First pre-filled glucagon pen, Tetris Pharma’s Ogluo®, now available in the UK for the treatment of severe hypoglycaemia
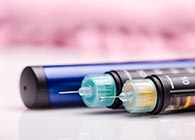
Tetris Pharma has announced that people living with diabetes in the UK now have access to Ogluo® (glucagon Pre-Filled Pen). This is the first ready-to-use, pre-mixed and pre-measured liquid glucagon injection to treat severe hypoglycaemia in adults, adolescents, and children aged two years and over.
Severe hypoglycaemia is currently treated with a glucagon treatment requiring eight stages to prepare and inject. When someone is unconscious or unable to take glucose orally and a number of steps to mix the glucagon powder and liquid prior is required, this is often not done successfully under pressure, which may delay recovery. Providing glucagon as a liquid in a new, easy-to-use auto-injection pen could make this process far simpler and mean greater freedom for people with diabetes and their families. In a simulated human factor study of 16 individuals both trained & untrained, only 31% of participants were successfully able to administer the glucagon solution. Ineffective treatment of hypoglycaemic episodes can substantially increase overall direct and indirect healthcare costs as a result of a patient’s need for emergency support and hospitalisation. The estimated total cost of a severe hypoglycaemic episode can be over £2,000.
Ogluo, unlike current glucagon emergency kits, is a reliable two-step administration of glucagon through a pre-filled pen in case of a severe hypoglycaemia. In a study, 99% of participants were successfully able to administer it. To help ensure the right dose, two pre-measured dosing options for adults and children are available. Sub-cutaneous glucagon has an established safety profile, with Ogluo being stable at room temperature, portable, ready-to-use with no visible needle, and can be stored for up to 27 months for the 1mg & 24 months for the 0.5mg.
Hypoglycaemia is responsible for an estimated 70,000–100,000 emergency callouts in the UK costing between £16m-£24m/annum. Severe hypoglycaemia occurs when blood glucose levels drop below 4mmol/L and is defined as requiring assistance from another person to treat it. Severe hypoglycaemia has an annual prevalence of 30–40% amongst people living with Type 1 diabetes and is the second most common cause of hospital admissions for drug-related adverse events.
Back to news archive