MHRA: Faulty Autopens recall
MHRA: Faulty Autopens recall
17th December 2014
People with diabetes who use insulin pens manufactured by Owen Mumford are today being warned by the Medicines and Healthcare products Regulatory Agency (MHRA) to check if they have an affected LOT. If they have a pen from the list of affected LOTs, then they should stop using them and receive a replacement at the earliest opportunity.
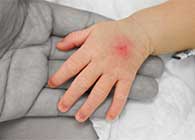
Owen Mumford has initiated a voluntary recall of affected LOTs and this is due to a dosage fault with the pens which could lead to hyperglycaemia if an underdose of insulin is given.
The fault with the four specific pens Autopen Classic 1 unit pen, Autopen Classic 2 unit pen, Autopen 24 1 unit pen and Autopen 24 unit 2 pen, can cause the dose selector to suddenly revert to zero which could lead to people receiving an underdose of insulin or no insulin at all.
The failure rate of these 4290 devices that have been distributed in the UK is 42%.To identify affected devices still within their packaging, look for the following packaging LOT code on the outer carton.
| Packaging lot code |
Product code |
Product name |
|
7VJ |
AN3810 |
3ml 1 Unit Autopen Classic |
|
7WB |
AN4200 |
Autopen 24 3ml 2 Unit |
|
7WC |
AN4210 |
Autopen 24 3ml 1 Unit |
|
7WD |
AN3800 |
3ml 2 Unit Autopen Classic |
|
8CN |
AN4200 |
Autopen 24 3ml 2 Unit |
|
8CP |
AN4200 |
Autopen 24 3ml 2 Unit |
|
8CR |
AN4210 |
Autopen 24 3ml 1 Unit |
|
8EL |
AN3800 |
3ml 2 Unit Autopen Classic |
|
8EM |
AN3810 |
3ml 1 Unit Autopen Classic |
|
8JK |
AN4210 |
Autopen 24 3ml 1 Unit |
|
8JM |
AN3800 |
3ml 2 Unit Autopen Classic |
|
8JN |
AN3810 |
3ml 1 Unit Autopen Classic |
|
8JP |
AN4210 |
Autopen 24 3ml 1 Unit |
|
8VV |
AN4200 |
Autopen 24 3ml 2 Unit |
|
8VW |
AN4200 |
Autopen 24 3ml 1 Unit |
To identify affected devices without packaging look for the following production LOT codes which can be found on the lower part of the pen body.
| Production lot code (stamped on pen) |
Product code |
Product name |
|
7RT |
AN3810 |
3ml 1 Unit Autopen Classic |
|
7PN |
AN4200 |
Autopen 24 3ml 2 Unit |
|
7PP |
AN4210 |
Autopen 24 3ml 1 Unit |
|
7RV |
AN3800 |
3ml 2 Unit Autopen Classic |
|
8KK |
AN3800 |
3ml 2 Unit Autopen Classic |
|
8XD |
AN4200 |
Autopen 24 3ml 2 Unit |
MHRA Clinical Director of Devices, Dr Neil McGuire said:
“It is vital that people check if they have these affected pens and should obtain an alternative device before stopping using them. People should seek a replacement at the earliest opportunity by contacting Owen Mumford at: technical.support@owenmumford.co.uk or visiting their pharmacist.
“Patients who experience unexpectedly raised blood glucose on self-testing or symptoms of hyperglycaemia, regardless of which insulin delivery device they are using should contact a healthcare professional immediately, if it does not respond to their usual rescue treatment.
“We continue to encourage people to report incidents involving medical devices to the MHRA via
https://yellowcard.mhra.gov.uk/ ”